1. Introduction
Trace metal clean seawater subsampling
Instructors: Yihua Cai and Liping Ye
In this practical session, participants will be instructed, imitating an onboard situation, how to handle in-line seawater filtration on Niskin-X bottles for trace elements and isotopes in a clean container.
Flow Injection Analysis (FIA)-chemiluminescence detection
Instructors: Yongming Huang and Kuanbo Zhou
This short practical training will be focused on the dissolved iron measurement by using flow Injection coupled to chemiluminescence (FIA-CL). The detailed principle of the FIA-CL analysis and cautions of sample handling during operation will be introduced. Working curves with different concentration gradients will be made during training. In addition, standard addition calibrations using a low-Fe seawater sample will also be performed. To assess the reproducibility of the method, a series of replicates to low-Fe seawater will be measured.
Multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS)
Instructors: Zhimian Cao and Yaojin Chen
Thanks to the plasma source and the multiple ion collector device, a high ionization efficiency and a simultaneous determination of separated ion beams are applied to the precise and accurate isotopic ratio measurements by MC-ICP-MS. It is widely used for determination of many isotopic systems in marine geochemistry, such as U, Th, Nd, Fe, Cd, Ca, Sr, Ba, Si, and many others.
The demo instrumentation is a Nu Plasma HR MC-ICP-MS which is featured by a double focusing magnetic-sector design. The structure of mass analyzer is based on the standard Nier-Johnson design, an electrostatic analyzer (ESA) positioned before a magnetic sector. Using the magnet, the ion beams are separated by mass and focused onto a Faraday detector array. The demo will present in the following order:
- Introduction of the system
- Tuning the instrument for a particular isotopic system
- Setting the data acquisition routine
- Writing the data calibration program
- Performing the data acquisition
- Viewing and analyzing the results
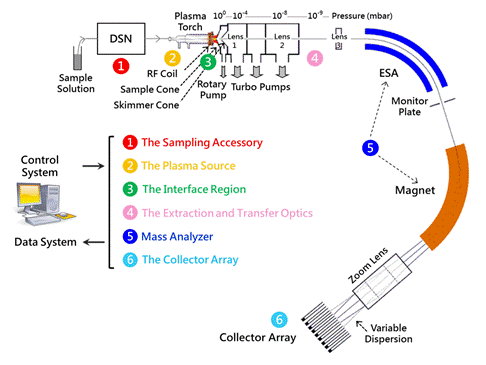
Fig. 1 Schematic of Nu Plasma HR (adapted from Nu Instruments)
Metal speciation
Instructors: George Luther III, Deli Wang and Rujun Yang
Voltammetry is the measurement of the current as a function of applied potential in conductive analyst, used in trace metal analysis and speciation such as seawater. Voltammetry can be adapted for a variety of experimental procedures depending on the electrode and conditions. Particularly using competitive ligand exchange/adsorptive cathodic stripping voltammetry, it is commonly used to measure low (pico and nanomolar) concentrations of trace metals (e.g., iron) and organic compounds, reaction kinetics and the ligand concentration and binding strength of a metal-ligand complex in marine chemistry. This practical session will then briefly present:
1) The hardware, and the NOVA or GPES software;
2) Demonstrating metal speciation experiments by voltammetry in lectures;
3) Setting up and running a measurement of trace metals from open ocean (e.g., iron);
4) Peak measurement and data interpretation
Solid state electrode fabrication & Polishing, electroplating and use
Instructors: George Luther III, Kunming Xu and Mustafa Yucel
Voltammetry measures redox chemical species by quantifying the electric currents of their redox reactions upon scanning a potential range at a certain rate. Here we introduce the solid state voltammetric electrode by electrodepositing mercury onto a gold electrode. The application of Hg-Au electrodes to the sediment-water interface has led to significant scientific advances. To quantify chemical species within sediments, the traditional procedure is to slice the sediment cores according to their depths and squeeze the samples mechanically for sediment pore-water, which are then analyzed using chemical titration or instrumental methods. These procedures normally disturb the sediments such as change the temperature, pressure, and other physical parameters of the samples and cannot achieve a high vertical resolution. Therefore, a preferable method is to insert a solid state electrode into the sediment depths for direct chemical concentration profiling.
This practical training session will teach hands-on skill of building solid state Hg-Au electrodes. The construction of the electrode will include electrode fabrication, electrode polishing and electroplating, and electrode use for redox species measurement. By adopting three-electrode cell and square wave voltammetry, the Hg-Au electrode can measure dissolved oxygen, Mn (II), Fe (II, III), S (-II) and other redox chemical species within a potential scan from –0.10V to –1.75V versus saturated calomel electrode (SCE). Demonstration experiments of square wave voltammetry will be conducted on a μAutolab II potentiostat with a hanging mercury drop electrode (HMDE) or a solid state electrode. The hardware interfaced to GPES software will be presented briefly. |